Understanding the regulation of tissue growth is of significant clinical interest, as it can shed light on processes ranging from tissue regeneration to inhibition of uncontrolled growth in cancer. We are using a computational modelling approach to investigate how mechanics can regulate tissue growth and shape formation. The model system we are utilising is the development of wing imaginal disc in Drosophila during larval stage, and the computational model is developed in parallel with novel experimental data generation within our group.
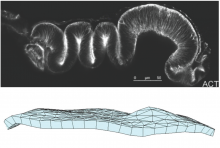
Wing disc is initially a flat tissue that folds over itself to form a sack like structure. During development, the growing wing disc forms a series of complex folds in 3D, which eventually define the wing blade and wing pouch regions, latter of which forms part of the fly¹s torso, and the connection between the wing and the body. We are investigating what are the factors that drive the formation of these folds, and once formed what are the contribution of these folds to further force accumulation and morphogenesis within the tissue. As the wing disc grows, we predict that differential proliferation at different regions of the tissue will lead to accumulation of forces. We are investigating if these accumulated forces would be sufficient to form the experimentally observed folds. If the force accumulation is not sufficient, we would like to identify what the necessary physical regulations, such as heterogeneities in physical properties of the tissue, or active local force generation, would be necessary. A second layer of complexity arises from the potential feedbacks mechanical forces would have on the local physical properties and growth rates. We aim to dissect these feedbacks with our computational approach, and explain the potential roles of the folds in later stages of morphogenesis.